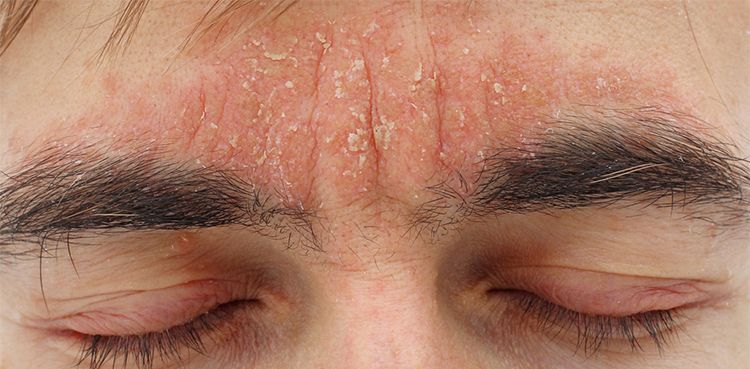
The US Food and Drug Administration (FDA) on Friday approved Arcutis Biotherapeutics’ drug for treating a skin condition called seborrheic dermatitis in individuals nine years of age and older.
Shares of Arcutis jumped 20% in extended trade to $2.94.
The health regulator’s nod makes roflumilast foam the first topical drug for treating moderate to severe seborrheic dermatitis with a new mechanism of action in over two decades, according to the company.
Seborrheic dermatitis, a common, chronic and recurrent inflammatory skin disease, affects more than 10 million people in the U.S., Arcutis said.
The drug is a foam-based formulation of the company’s roflumilast cream 0.3%, sold as Zoryve, which is approved in the U.S. as a topical treatment of plaque psoriasis in patients 6 years of age and older.
The drug met its primary study goal in the late-stage trial with a success rate of 79.5% on the 5-point assessment scale compared with 58.0% in those treated with the vehicle, which is similar to a placebo.
The formulation, designed to overcome the challenges of delivering topical drugs in hair-bearing areas of the body, also showed meaningful improvement over the vehicle arm in disease symptoms, including itch, scaling, and redness.
Arcutis said it plans to commercially launch Zoryve by the end of January.
Chief Commercial Officer Todd Edwards had earlier said that two of the top three U.S. pharmacy benefit managers are expected to cover the product on approval.
Guggenheim analyst Seamus Fernandez estimates revenue of $30.8 million and $94 million in 2024 and 2025, respectively, for the seborrheic dermatitis indication.
A low dose version of the roflumilast cream to treat atopic dermatitis in adults and children down to age 6 is under the FDA’s review, with the regulator set to make a decision in July.
from Health News - Latest breaking Health News - ARY NEWS https://ift.tt/5IzYJXB


0 Comments