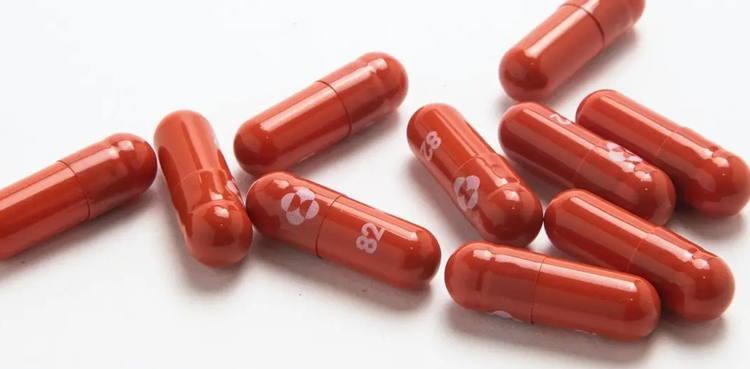
China has granted conditional approval for the import of Merck’s COVID-19 treatment Molnupiravir, China’s medical products regulator said on Friday.
Molnupiravir, developed by Merck which is also known as MSD outside the United States and Canada, is approved to be used in adult patients who have mild to medium COVID infection and a high risk of progressing to severe cases, according to China’s National Medical Products Administration.
Here is what you need to know about the antiviral pill.
Molnupiravir (MK-4482/EIDD-2801) is an investigational, orally administered form of a potent ribonucleoside analog that inhibits the replication of SARS-CoV-2, the causative agent of COVID-19. Molnupiravir has been shown to be active in several preclinical models of SARS-CoV-2, including for prophylaxis, treatment, and prevention of transmission. Additionally, pre-clinical and clinical data have shown molnupiravir to be active against the most common SARS-CoV-2 variants. Molnupiravir was invented at Drug Innovations at Emory (DRIVE), LLC, a not-for-profit biotechnology company wholly owned by Emory University, and is being developed by Merck & Co., Inc. in collaboration with Ridgeback Biotherapeutics. Ridgeback received an upfront payment from Merck and also is eligible to receive contingent payments dependent upon the achievement of certain developmental and regulatory approval milestones. Any profits from the collaboration will be split between the partners equally. Since licensed by Ridgeback, all funds used for the development of molnupiravir have been provided by Merck and by Wayne and Wendy Holman of Ridgeback.
from Health News - Latest breaking Health News - ARY NEWS https://ift.tt/FextN9W


0 Comments